Stephen M. Stahl, MD, PhD
CNS Spectr. 2007;12(10):739-7 Faculty Affiliation and DisclosuresDr. Stahl is adjunct professor of psychiatry in the Department of Psychiatry at the University of California-San Diego in La Jolla.Disclosures: Dr. Stahl receives grant/research support from AstraZeneca, Biovail, Bristol-Myers Squibb, Cephalon, Cyberonics, Eli Lilly, Forest, GlaxoSmithKline, Janssen, Neurocrine Bioscience, Organon, Pfizer, Sepracor, Shire, Somaxon, and Wyeth; is a consultant to Acadia, Amylin, Asahi, AstraZeneca, Biolaunch, Biovail, Boehringer-Ingelheim, Bristol-Myers Squibb, Cephalon, CSC Pharma, Cyberonics, Cypress Bioscience, Eli Lilly, Epix, Fabre Kramer, Forest, GlaxoSmithKline, Jazz, Neurocrine Bioscience, Neuromolecular, Neuronetics, Nova Del Pharma, Novartis, Organon, Otsuka, PamLab, Pfizer, Pierre Fabre, Sanofi Synthelabo, Schering Plough, Sepracor, Shire, Solvay, Somaxon, Takeda, Tetragenix, and Wyeth; and is on the speaker’s bureau of Pfizer.Email Dr. Stahl: vj@mblcommunications.com.
Activated Folate-MTHF from Thorne Research Available
Denise Lane-Acupuncturist
New Trend in Psychopharmacology
Folate deficiency may increase the risk of depression and reduce the action of antidepressants. Individuals with an inherited polymorphism that reduces the efficiency of folate formation may be at high risk for folate deficiency and for major depression. Antidepressant effects have been reported when antidepressants are augmented with folic acid, folinic acid, or the centrally active L-methylfolate (known formally as (6(S)-5-methyltetrahydrofolate [MTHF]), particularly in depressed patients with folate deficiency whose major depressive episodes have failed to respond to antidepressants. The putative mechanism of action of MTHF as an augmenting agent to antidepressants is that it acts as a trimonoamine modulator (TMM), enhancing the synthesis of the three monoamines: dopamine (DA), norepinephrine (NE), and serotonin (5-HT), resulting in a boost to the efficacy of antidepressants.
Folate and Depression
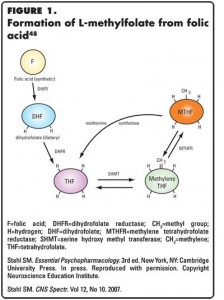
A substantial body of literature suggests that depression is associated with folate deficiency,1-31 and that patients with folate deficiency either experience a later onset of action,2,19 lesser improvement,4,8,9,13 a more severe depressive episode,8,19-21 or higher chances of relapse14 when taking antidepressants. The link between folate and depression is supported by observations that a common genetic variant of an enzyme that reduces one’s ability to convert folate to its centrally active metabolite, MTHF, specifically, the C677T variant of the enzyme methylene tetrahydrofolate reductase, is more common in patients with depression.15,26-28 In this article and in the figures, the centrally active metabolite of folate will be referred to either as L-methylfolate or as MTHF.

The formation of MTHF from foods containing dihydrofolate or enriched with synthetic folic acid is shown in Figure 1. The enzyme methylene tetrahydrofolate reductase forms this centrally active metabolite, MTHF, in the final step of this process (Figure 1). The link between folate and depression is also supported by numerous studies suggesting that folic acid,16,17 folinic acid,31 or MTHF18,32-35 either have antidepressant action or enhance the therapeutic benefits of antidepressants or lithium.
How Does L-methylfolate Act as an Antidepressant?
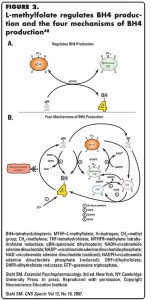
Antidepressants boost the actions of one or more of the three monoamines: DA, NE, and/or 5-HT. MTHF acts as an important regulator of a critical co-factor for trimonoamine neurotransmitter synthesis. This co-factor is known as tetrahydrobiopterin (BH4) (Figure 2A).36-48 By boosting trimonoamine neurotransmitter synthesis via enhancement of BH4, MTHF presumably is able to augment the antidepressant actions of known antidepressants.
Because BH4 is a critical enzyme co-factor, there are several mechanisms that lead to its formation (Figure 2B).36-48 BH4 can be formed from de novo synthesis, from guanosine triphosphate, or recycled via the enzyme dihydropteridine reductase (Figure 2B).38,39,44 BH4 formation that is linked to folate metabolism includes recycling either from the actions of methylene tetrahydrofolate reductase (mentioned above)44,45 or from the actions of dihydrofolate reductase (Figure 2B).37,43
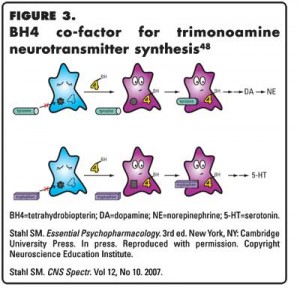
The trimonoamine synthetic enzymes that require BH4 as a co-factor are tryptophan hydroxylase, the rate-limiting enzyme for 5-HT synthesis and tyrosine hydroxylase, the rate-limiting enzyme for DA and NE synthesis (Figure 3).36,39,42 MTHF is thus considered to be a TMM due to its role as an indirect regulator of trimonoamine neurotransmitter synthesis and monoamine concentrations.48
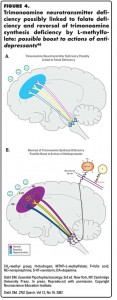
As previously mentioned, numerous studies suggest that low plasma, red blood cell, and/or cerebrospinal fluid levels of MTHF folate precursors (Figure 1) may be associated with depression in some patients (Figure 4A).1-30 Since MTHF indirectly regulates monoamine levels (Figures 2 and 3),36-48 low central nervous system levels of MTHF could lead to reduced activity of trimonoaminergic neurotransmitter-synthesizing enzymes, causing monoamine deficiency (Figure 4A), consistent with the monoamine hypothesis of depression.46-48
Studies16-35 have now shown that administration of folate, MTHF, or another folate derivative known as folinic acid (Leucovorin), can augment the therapeutic efficacy of antidepressants in patients with major depressive disorder who fail to respond adequately to their antidepressant, and who may or may not have measurable folate deficiency. Current research thus suggests that MTHF may be indicated for depressed patients with low plasma, red blood cell, and/or cerebrospinal fluid folate levels, and who have not responded adequately to antidepressants (Figure 4B).16-35 Theoretically, many patients with inadequate responses to a monoamine-enhancing antidepressant, even if there is no folate deficiency, could benefit from MTHF treatment that elevates their BH4 levels (Figure 1), if it led to enhancement of their trimonoamine neurotransmitter synthesis (Figure 3; also compare Figures 4A and 4B).36-48
Why L-methylfolate Rather Than Folic Acid for Depression?
Folate is one of the 13 essential vitamins. Dihydrofolate, a mixture of polyglutamates (ie, a number of glutamatic acid entities) is the form of folate obtained from dietary intake of green vegetables, yeast, liver, kidney, and egg yolk. Folic acid is the synthetic form of folate contained in over-the-counter vitamin supplements (usually mixed with several other vitamins and nutrients and present in low doses). Folic acid is also the synthetic form of folate contained in prescriptions written by a licensed practitioner in higher doses for medical use.
Dihydrofolate and folic acid are converted to monoglutamate entites by the enzyme alpha-L-glutamyl transferase in the intestinal wall as they are absorbed.47 Once absorbed, monoglutamate entities are converted to MTHF, the form of folate that passes into the brain and is utilized by trimonoamine neurons to facilitate neurotransmitter synthesis (Figures 1 and 4B). Normally, ingesting folate from dihydrofolate in the diet or from folic acid in synthetic supplements will result in adequate delivery of MTHF levels to the brain, especially in those individuals with the more efficient genotype (C677C) producing up to 100% of the enzyme methylene tetrahydrofolate reductase and who do not have depression.
However, robust levels of MTHF in the brain, which may be necessary to maximize the chances of boosting trimonoamine neurotransmitter synthesis (Figures 2–4), are more likely attained after administration of MTHF rather than folic acid (Figure 4B). Thus, administration of MTHF may have significant advantages over administration of folic acid as a TMM to augment antidepressants in depressed patients who do not respond adequately to their antidepressant treatment. Such patients may or may not be folate deficient, may or may not have the inefficient form of the genotype (C677T, T677T) producing 35% to 71% of the MTHF enzyme, and may or may not be taking various anticonvulsant mood stabilizers that interfere with folic acid absorption or MTHF formation, such as lamotrigine, carbamazepine, and others (Figure 4B). Further research is needed to identify those depressed patients most likely to respond to MTHF augmentation, including studies of both unipolar and bipolar depression.
In terms of what is known about treatment with folic acid versus MTHF, it may take as much as 7 mg of oral folic acid to generate the same plasma levels of MTHF as giving 1 mg of oral MTHF.49 How much folic acid is this? The recommended daily allowance of folic acid from food or dietary supplements is 0.4 mg (0.8 mg for pregnant women); over-the-counter multivitamin supplements typically provide between 0.25 and 1 mg of folic acid; normal “prescription strength” folic acid is 1 mg pure folic acid; high-dose prescription folic acid for treating pregnant women to reduce the risk of neural tube defects is between 4 mg and 5 mg. By comparison, the lowest dose of MTHF studied in depression to augment antidepressant treatment is 7.5 mg, roughly equivalent to 52 mg of folic acid.32 Although high doses of folic acid can be administered orally, the precursors of MTHF may compete with MTHF for entry into brain by binding to folate transport receptors, limiting the amount of MTHF that can enter the brain (Figure 4B).50-52 Thus, high doses of MTHF are likely to provide substantially more active MTHF moiety to the brain than high doses of folic acid. The exact dose of MTHF to treat depression is not fully determined, but since MTHF works indirectly to boost monoamine synthesis, high doses are likely to be necessary to optimize this action.
Additionally, high doses of MTHF may be more appropriate than high doses of folic acid because MTHF is less likely to mask a vitamin B12 deficiency.53-55 That is, when folic acid is administered, it can be metabolized and utilized for DNA biosynthesis even in vitamin B12 deficient cells, thus masking the anemia from a vitamin B12 deficiency. B12 deficiency decreases the methyl donor S-adenosyl-methionine (SAMe), which activates the enzyme MTHF reductase and this traps MTHF away from DNA synthesis, making it unlikely that the administration of MTHF will mask an anemia.53-56
What is a Medical Food?
MTHF is marketed in the United States as a “medical food” also called Deplin, which contains 7.5 mg L-methylfolate and is available by prescription only. According to the Food and Drug Administration, a medical food is different both from a drug and from a food, and is defined as a food that is formulated to be consumed orally
“…under the supervision of a physician and which is intended for the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation.”57
Medical foods are required when dietary management cannot achieve the specific nutrient requirements. Treatment with MTHF seems to be safe, apparently has few if any side effects, and is generally less expensive than augmenting with a second antidepressant. Further research is necessary to determine the exact priority this approach should be given in treatment algorithms for major depression.
S-adenosyl-methionine, L-methylfolate, and Methylation
MTHF may have additional actions on monoamine neurotransmitter metabolism through another mechanism, namely, its well-known ability to regulate methylation reactions (Figure 5).37 Another agent possibly useful for augmenting antidepressants in patients with inadequate responses is SAMe, which shares with MTHF the ability to regulate methylation (Figure 5).58,59 Both MTHF and SAMe may therefore impact the regulation of various critical 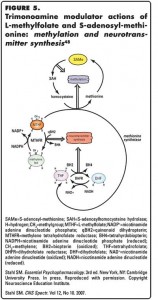 components of monoamine neurotransmitter activity not only by indirect modulation of neurotransmitter synthesis by promoting the synthesis of BH4 enzymatic cofactor, but also by modulating catabolic enzymes, monoamine transporters, and neurotransmitter receptors via methylation and its downstream effects (Figure 5).60-65 These complex mechanisms are under active investigation to determine how the natural products and putative TMMs, MTHF and SAMe, may contribute to the treatment of depression.
components of monoamine neurotransmitter activity not only by indirect modulation of neurotransmitter synthesis by promoting the synthesis of BH4 enzymatic cofactor, but also by modulating catabolic enzymes, monoamine transporters, and neurotransmitter receptors via methylation and its downstream effects (Figure 5).60-65 These complex mechanisms are under active investigation to determine how the natural products and putative TMMs, MTHF and SAMe, may contribute to the treatment of depression.
Conclusion
L-methylfolate, also known as MTHF (methyltetrahydrofolate), acts as a trimonoamine modulator to boost the synthesis of the three monoamines: DA, NE, and 5-HT. This action may provide antidepressant efficacy when L-methylfolate is given as an augmenting agent to depressed patients unresponsive to traditional antidepressants. L-methylfolate may be especially useful in depressed patients who have the genotype coding for an enzyme that causes inefficient synthesis of L-methylfolate, and for those individuals who are folate deficient, including patients whose folate deficiency is secondary to the administration of various anticonvulsant mood stabilizers.
References
1. Coppen A, Bolander-Gouaille C. Treatment of depression: time to consider folic acid and vitamin B12. J Psychopharmacol. 2005;19:59-65.
2. Papakostas GI, Petersen T, Lebowitz BD, et al. The relationship between serum folate, vitamin B12, and homocysteine levels in major depressive disorder and the timing of improvement with fluoxetine. Int J Neuropsychopharmacol. 2005;8:523-528.
3. Tolmunen T, Voutilainen S, Hintikka J, et al. Dietary folate and depressive symptoms are associated in middle-aged Finnish men. J Nutr. 2003;133:3233-3236.
4. Alpert M, Silva RR, Pouget ER. Prediction of treatment response in geriatric depression from baseline folate level: interaction with an SSRI or a tricyclic antidepressant. J Clin Psychopharmacol. 2003;23:309-313.
5. Morris MS, Fava M, Jacques PF, Selhub J, Rosenberg IH. Depression and folate status in the US Population. Psychother Psychosom. 2003;72:80-87.
6. Bottiglieri T, Laundy M, Crellin R, Toone BK, Carney MW, Reynolds EH. Homocysteine, folate, methylation, and monoamine metabolism in depression. J Neurol Neurosurg Psychiatry. 2000;69:228-232.
7. Alpert JE, Fava M. Nutrition and depression: the role of folate. Nutr Rev. 1997;55:145-149.
8. Fava M, Borus JS, Alpert JE, Nierenberg AA, Rosenbaum JF, Bottiglieri T. Folate, vitamin B12, and homocysteine in major depressive disorder. Am J Psychiatry. 1997;154:426-428.
9. Wesson VA, Levitt JA, Joffe RT. Change in folate status with antidepressant treatment. Psychiatry Res. 1994;53:313-322.
10. Coppen A, Swade C, Jones SA, Armstrong RA, Blair JA, Leeming RJ. Depression and tetrahydrobiopterin: the folate connection. J Affect Disord. 1989;16:103-107.
11. Abou-Saleh MT, Coppen A. Serum and red blood cell folate in depression. Acta Psychiatr Scand. 1989;80:78-82.
12. Abou-Saleh MT, Coppen A. The biology of folate in depression: implications for nutritional hypotheses of the psychoses. J Psychiatr Res. 1986;20:91-101.
13. Papakostas GI, Petersen T, Mischoulon D, et al. Serum folate, vitamin B12, and homocysteine in major depressive disorder, Part 1: predictors of clinical response in fluoxetine-resistant depression. J Clin Psychiatry. 2004;65:1090-1095.
14. Papakostas GI, Petersen T, Mischoulon D, et al. Serum folate, vitamin B12, and homocysteine in major depressive disorder, Part 2: predictors of relapse during the continuation phase of pharmacotherapy. J Clin Psychiatry. 2004;65:1096-1098.
15. Gilbody S, Lewis S, Lightfoot T. Methylene tetrahydro folate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. Am J Epidemiol. 2006;165:1-13.
16. Coppen A, Bailey J. Enhancement of the antidepressant action of fluoxetine by folic acid: a randomised, placebo controlled trial. J Affect Disord. 2000;60:121-130.
17. Coppen A, Chaudhry C, Swade C. Folic acid enhances lithium prophylaxis. J Affect Disord. 1986;10:9-13.
18. Passeri M, Cucinotta D, Abate G, et al. Oral 5’-methyltetrahydrofolic acid in senile organic mental disorders with depression: results of a double-blind multicenter study. Aging (Milano). 1993;5:63-71.
19. Carney MW, Chary TK, Laundy M. Red cell folate concentrations in psychiatric patients. J Affect Disord. 1990;19:207-213.
20. Reynolds EH, Preece JM, Bailey J, Coppen A. Folate deficiency in depressive illness. Br J Psychiatry. 1970;117:287-292.
21. Lerner V, Kanevsky M, Dwolatzky T, Rouach T, Kamin R, Miodownik C. Vitamin B12 and folate serum levels in newly admitted psychiatric patients. Clin Nutr. 2006;25:60-67.
22. Sachdev PS, Parslow RA, Lux O, et al. Relationship of homocysteine, folic acid and vitamin B12 with depression in a middle-aged community sample. Psychol Med. 2005;35:529-538.
23. Levitt AJ, Joffe RT. Folate, B12, and life course of depressive illness. Biol Psychiatry. 1989;25:867-872.
24. Wesson VA, Levitt AJ, Joffe RT. Change in folate status with antidepressant treatment. Psychiatry Res. 1994;53:313-322.
25. Wilkinson A, Anderson D, Abou-Saleh M, et al. 5-Methyltetrahydrofolate level in the serum of depressed subjects and its relationship to the outcome of ECT. J Affect Disord. 1994;32:163-168.
26. Kelly CB, McDonnell AP, Johnston TG, et al. The MTHFR C677T polymorphism is associated with depressive episodes in patients from Northern Ireland. J Psychopharmacol. 2004;18:567-571.
27. Lewis SJ, Lawlor DA, Davey Smith G, et al. The thermolabile variant of MTHFR is associated with depression in the British Women’s Heart and Health Study and a meta-analysis. Mol Psychiatry. 2006;11:352-360.
28. Arinami T, Yamada N, Yamakawa-Kobayashi K, et al. Methylenetetrahydrofolate reductase variant and schizophrenia/depression. Am J Med Genet. 1997;74:526-528.
29. Gilbody S, Lightfoot T, Shelton T. Is low folate a risk factor for depression? A meta-analysis and exploration of heterogeneity. J Epidemiol Community Health. 2007;61:631-637.
30. Blair JA, Barford PA, Morar C, et al. Tetrahydrobiopterin metabolism in depression. Lancet. 1984;2:163.
31. Alpert JE, Mischoulon D, Rubenstein GE, Bottonari K, Nierenberg AA, Fava M. Folinic acid (Leucovorin) as an adjunctive treatment for SSRI-refractory depression. Ann Clin Psychiatry. 2002;14:33-38.
32. Guaraldi GP, Fava M, Mazzi F, la Greca P. An open trial of methyltetrahydrofolate in elderly depressed patients. Ann Clin Psychiatry. 1993;5:101-105.
33. Godfrey PS, Toone BK, Carney MW, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336:392-395.
34. Reynolds EH, Crellin R, Bottiglieri T, Laundy M, Toone BK, Carney M. Methylfolate as monotherapy in depression: a pilot randomized controlled trial. Abstract presented at: the Annual Meeting of the Royal College of Psychiatrists. July 24-27, 1992; Edinburgh, UK.
35. Di Palma C, Urani R, Agricola R, Giorgetti V, Dalla Verde G. Is Methylfolate effective in relieving major depression in chronic alcoholics? A hypothesis of treatment. Curr Ther Res. 1994;55:559-568.
36. Meller E, Friedhoff AJ. 5-methyltetrahydrofolate and metabolism of biogenic amines; In: Botez MI, Reynolds EH, eds. Folic Acid in Neurology, Psychiatry and Internal Medicine. New York, NY: Raven Press; 1979:157-164.
37. Bottiglieri T, Hyland K, Laundry M, et al. Folate deficiency, biopterin and monoamine metabolism in depression. Psychol Med. 1992;22:871-876.
38. Ponzone A, Spada M, Ferraris S, Dianzani I, deSanctis L. Dihydropteridine reductase deficiency in man: from biology to treatment. Med Res Rev. 2004;24:127-150.
39. Goldstein DS, Hahn S-H, Holmes C, et al. Monoaminergic effects of folinic acid, l-dopa and 5-hydroxytryptophan in dihydropteridine reductase deficiency. J Neurochem. 1995;64:2810-2813.
40. Lucock MD, Green M, Levene MI. Methylfolate modulates potassium evoked neuro-secretion: evidence for a role at the pteridine cofactor level of tyrosine 3-hydroxylase. Neurochem Res. 1995;20:727-736.
41. Niederwieser A. Inborn errors of Pterin metabolism. In: Botez MI, Reynolds EH, eds. Folic Acid in Neurology, Psychiatry and Internal Medicine. New York, NY: Raven Press; 1979:349-384.
42. Turner AJ. The relationship between brain folate and monoamine metabolism. In: Botez MI, Reynolds EH, eds. Folic Acid in Neurology, Psychiatry and Internal Medicine. New York, NY: Raven Press; 1979:165-177.
43. Hamon CGB, Blair JA, Barford PA. The effect of tetrahydrofolate on tetrahydrobipterin metabolism. J Ment Defic Res. 1986;30:179-183.
44. Matthews RG, Kaufman S. Characterization of the dihydropterin reductase activity of pig liver methylenetetrahydrofolate reductase. J Biol Chem. 1980;255:6014-6017.
45. Vanoni MA, Ballou DP, Matthews RG. Methylenetetrahydrofolate reductase. Steady state and rapid reaction studies on the NADPH-methylenetetrahydrofolate, NADPH-menadione, and methyltetrahydrofolate-menadione oxidoreductase activities of the enzyme. J Biol Chem. 1983;258:11510-11514.
46. Farrar G, Blair JA. Pterins in depression: a modified monoamine hypothesis. In: Copeland JR, Abou-Saleh MT, Blazer DG, eds. Principles and Practice of Geriatric Psychiatry. Hoboken, NJ: John Wiley and Sons; 1994:543-546.
47. Paul RT, McDonnell AP, Kelly CB. Folic acid: neurochemistry, metabolism and relationship to depression. Hum Psychopharmacol. 2004;19:477-488.
48. Stahl SM. Essential Psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; In press.
49. Willems FF, Boers GH, Blom HJ, Aengevaeren WR, Verheugt FW. Pharmacokinetic study on the utilisation of 5-methyltetrahydrofolate and folic acid in patients with coronary artery disease. Br J Pharmacol. 2004;141:825-830.
50. Smith I, Hyland K, Kendall B. Clinical role of pteridine therapy in tetrahydrobiopterin deficiency. J Inherit Metab Dis. 1985;8(suppl 1):39-45.
51. Wu D, Pardridge WM. Blood-brain barrier transport of reduced folic acid. Pharm Res.1999;16:415-419.
52. Spector R, Lorenzo AV. Folate transport in the central nervous system. Am J Physiol. 1975;229:777-782.
53. Scott JM, Weir DG. The methyl folate trap. A physiological response in man to prevent methyl group deficiency in kwashiorkor (methionine deficiency) and an explanation for folic-acid induced exacerbation of subacute combined degeneration in pernicious anaemia. Lancet. 1981;2:337-340.
54. Kelly P, McPartlin J, Goggins M, Weir D, Scott JM. Unmetabolized folic acid in serum: acute studies in subjects consuming fortified food and supplements. Am J Clin Nutr. 1997;65:1790-1795.
55. Morris MS, Jacques PF, Rosenberg IH, Selhub J. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am J Clin Nutr. 2007;85:193-200.
56. Akoglu B, Schrott M, Bolouri H, et al. The folic acid metabolite L-5-methyltetrahydrofolate effectively reduces total serum homocysteine level in orthotopic liver transplant recipients: a double-blind placebo-controlled study. Eur J Clin Nutr. In press.
57. Food and Drug Administration/Center for Food Safety and Applied Nutrition. Food Safety and Applied Nutrition, Medical Foods. Available at: http://www.cfsan.fda.gov/~dms/medfguid.html. Accessed September 13, 2007.
58. Mischoulon D, Fava M. Role of S-adenosyl-L-methionine in the treatment of depression: a review of the evidence. Am J Clin Nutr. 2002;76(suppl):1158S-1161S.
59. Bottiglieri T. S-Adenosl-L-methionine (SAMe): from the bench to the bedside – molecular basis of a pleiotrophic molecule. Am J Clin Nutr. 2002;76(suppl):1151S-1157S.
60. Vafai SB, Stock JB. Protein phosphatase 2A methylation: a link between elevated plasma homocysteine and Alzheimer’s disease. FEBS Lett. 2002;518:1-4.
61. Mann SP, Hill MW. Activation and inactivation of striatal tyrosine hydroxylase: the effects of pH, ATP, cyclic AMP, S-adenosylmethionine and S-adenosylhomocysteine. Biochem Pharmacol. 1983;32:3369-3374.
62. Curcio M, Catto E, Stramentinoli G, Algeri S. Effect of S-adenosyl-l-methionine on serotonin metabolism in rat brain. Prog Neuropsychopharmacol. 1978;2:65-71.
63. Ruck A, Kramer S, Metz J, Brennan MJ. Methyltetrahydrofolate is a potent and selective agonist for kainic acid receptors. Nature. 1980;287:852-853.
64. Bauman AL, Apparsundaram S, Ramamoorthy S, Wadzinski BE, Vaughan RA, Blakely RD. Cocaine and antidepressant-sensitive biogenic amine transporters exist in regulated complexes with protein phosphatase 2A. J Neurosci. 2000;20:7571-7578.
65. Ramamoorthy S, Gioivanetti E, Qian Y, Blakely RD. Phosphorylation and regulation of antidepressant-sensitive serotonin transporters. J
Novel Therapeutics for Depression: L-methylfolate as a Trimonoamine Modulator and Antidepressant-Augmenting Agent
Inflammation, Glutamate, and Glia in Depression: A Literature Review
Beyond the Dopamine Hypothesis to the NMDA Glutamate Receptor Hypofunction Hypothesis of Schizophrenia
Bipolar Depression: Best Practices for the Outpatient
Home | About Us | Contact Us | Our Mission | Journal CME | CME Enduring Materials
Clinical Supplements | Clinical Columns/News | Disease States | Advisory Board | Services & Online Store
Partner/Alliances | © 2012
Privacy Policy | Site Map | Web design and development by Spindustry Interactive